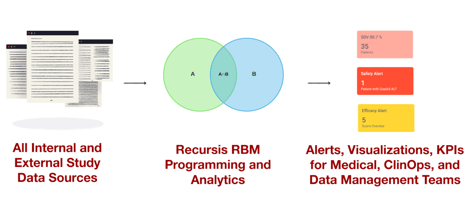
Regulatory agencies don't just review your efficacy data. They thoroughly examine your patient...
What Data Can Most Influence Clinical Trial Success or Failure?
After years of working across oncology, immunology, cardiovascular, infectious disease, and neurological trials, I've witnessed many studies succeed and fail. Through these experiences, one pattern has emerged with remarkable consistency: patient enrollment data is the bedrock of any successful clinical trial.
Yet despite its critical importance, most studies fail to leverage enrollment data properly, missing opportunities that could make or break their success. This oversight has profound implications for drug development timelines, costs, and ultimately, patient outcomes.
The Hidden Impact of Poor Enrollment Data Management
From what I've observed in my consulting work, poor enrollment data management consistently adds months to trial timelines. When you consider that drug development represents billions in investment, every delay compounds costs exponentially. More importantly, delays mean patients wait longer for potentially life-saving treatments.
The problem isn't just operational: it's strategic. Organizations that treat enrollment data as merely an administrative requirement rather than a strategic asset consistently underperform in trial execution and regulatory success.
What Makes Enrollment Data So Critical?
Enrollment data encompasses far more than basic demographics. It includes medical history, genetic profiles, disease characteristics, and biomarker data that collectively influence every aspect of trial outcomes. This information doesn't just describe your patient population; it predicts whether your trial will succeed or fail.
In my experience working with clinical teams across therapeutic areas, I've seen how proper enrollment data management can transform trial trajectories. Conversely, I've witnessed how inadequate data collection can derail even the most promising compounds.
Looking Ahead
I'll be sharing ideas and insights on how to transform enrollment data from a compliance checkbox into clinical intelligence. These aren't theoretical frameworks: they're battle-tested strategies developed through years of hands-on clinical data projects.
The goal is simple: help your organization avoid the common pitfalls that cost millions and delay life-changing treatments from reaching patients who need them most.